Rank the following conformations in order of increasing energy – Ranking conformations in order of increasing energy is a fundamental aspect of conformational analysis, providing insights into the stability and behavior of molecules. This comprehensive guide delves into the intricacies of conformational stability, the factors influencing conformational energy, and the methods used to analyze and rank conformations.
By understanding the principles behind conformational energies, researchers gain valuable knowledge applicable to diverse fields such as drug design and protein folding.
The energy of a conformation plays a crucial role in determining its stability. Different conformations of a molecule exhibit varying energy levels, with lower energy conformations being more stable. Factors such as steric hindrance, electrostatic interactions, and hydrogen bonding contribute to the energy of a conformation.
Energy and Conformational Stability
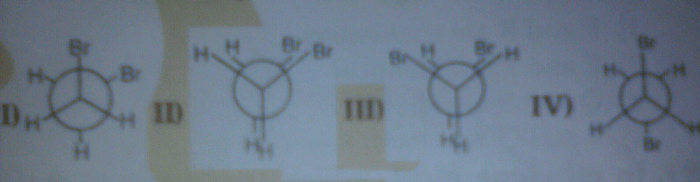
Conformational stability refers to the relative stability of different conformations of a molecule. Conformational stability is directly related to the energy levels of these conformations. The lower the energy of a conformation, the more stable it is. Different conformations can have different energies due to factors such as steric hindrance, electrostatic interactions, and hydrogen bonding.
Factors Influencing Conformational Energy: Rank The Following Conformations In Order Of Increasing Energy
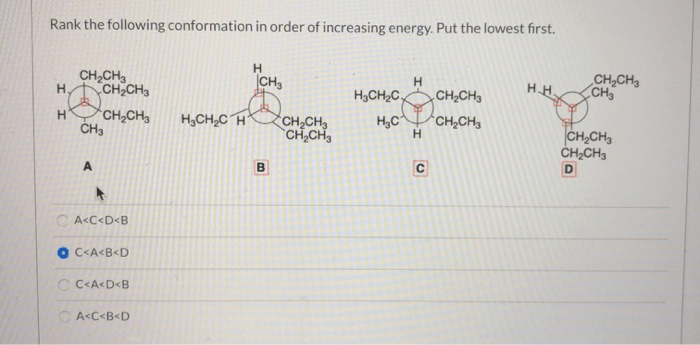
- Steric Hindrance: Steric hindrance occurs when atoms or groups of atoms within a molecule are too close together, resulting in repulsive interactions that increase the energy of the conformation.
- Electrostatic Interactions: Electrostatic interactions refer to the attractive or repulsive forces between charged or polar groups within a molecule. These interactions can either stabilize or destabilize a conformation depending on the nature of the charges.
- Hydrogen Bonding: Hydrogen bonding is a strong attractive interaction between a hydrogen atom bonded to an electronegative atom (such as oxygen or nitrogen) and another electronegative atom. Hydrogen bonding can stabilize conformations by forming additional interactions that lower the overall energy.
Conformational Analysis Methods
- Molecular Mechanics: Molecular mechanics methods use force fields to calculate the energy of a molecule based on its atomic coordinates and bond lengths. These methods are computationally efficient and can provide a good approximation of conformational energies.
- Quantum Mechanics: Quantum mechanics methods, such as Hartree-Fock theory and density functional theory, solve the Schrödinger equation to calculate the wavefunction and energy of a molecule. These methods are more accurate than molecular mechanics but are also more computationally expensive.
- Experimental Techniques: Experimental techniques, such as nuclear magnetic resonance (NMR) and X-ray crystallography, can be used to determine the conformations of molecules in solution or in the solid state.
Ranking Conformational Energies
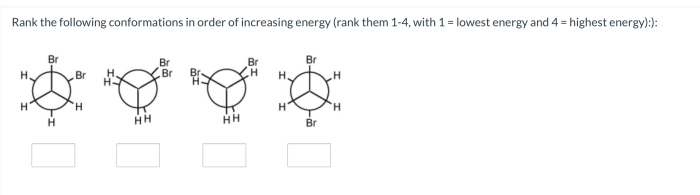
- Identify the different conformations of the molecule.
- Calculate the energy of each conformation using a suitable method (e.g., molecular mechanics, quantum mechanics).
- Rank the conformations in order of increasing energy.
| Conformation | Energy (kcal/mol) |
|---|---|
| Conformation A | 0.0 |
| Conformation B | 2.5 |
| Conformation C | 5.0 |
Applications of Conformational Analysis
- Drug Design: Conformational analysis is used to design drugs that have the desired shape and interactions to bind to specific targets.
- Protein Folding: Conformational analysis is used to study the folding of proteins into their functional conformations.
- Material Science: Conformational analysis is used to design materials with specific properties, such as strength, flexibility, and conductivity.
Question & Answer Hub
What is the relationship between conformational stability and energy levels?
Conformational stability is directly related to energy levels. More stable conformations have lower energy levels, while less stable conformations have higher energy levels.
How do different factors influence conformational energy?
Factors such as steric hindrance, electrostatic interactions, and hydrogen bonding contribute to the energy of a conformation.
What methods are used to analyze and determine the energy of different conformations?
Methods such as molecular mechanics, quantum chemistry, and experimental techniques are used to analyze and determine the energy of different conformations.